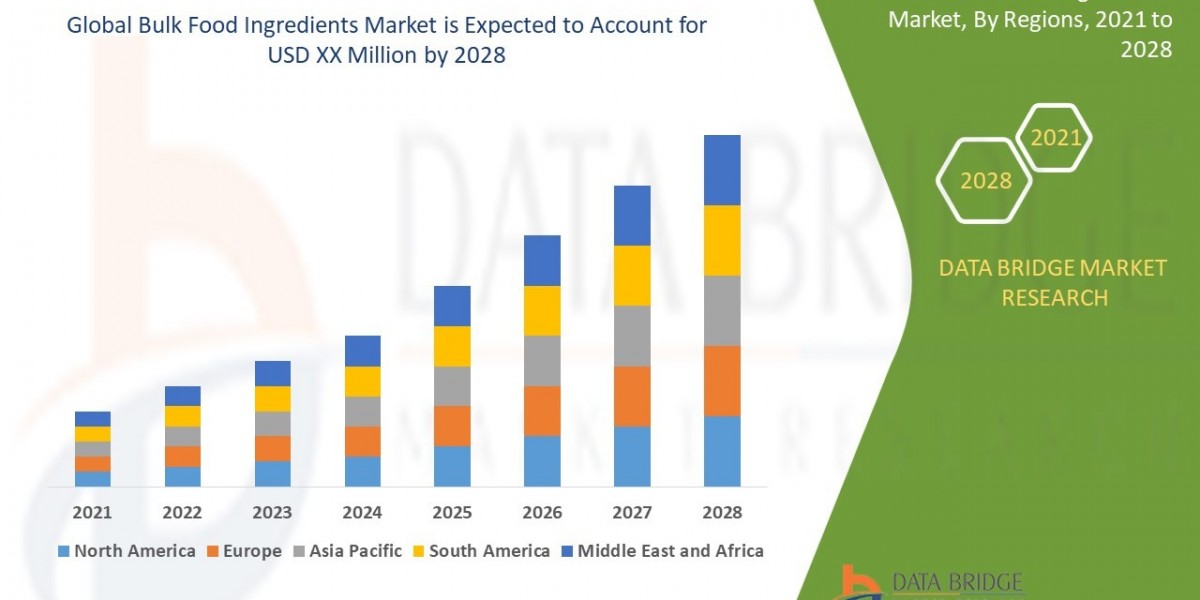
The global Opioid Withdrawal Treatment market is witnessing a critical period of growth and innovation, driven by the persistent opioid crisis and a growing emphasis on effective, accessible care. Currently estimated at USD 856.9 million in 2025, the market is poised for significant expansion, projected to reach approximately USD 1,747.0 million by 2035, demonstrating a robust Compound Annual Growth Rate (CAGR) of 7.4%. This upward trend reflects a concerted effort across healthcare systems, pharmaceutical companies, and governments to address the complex challenges of opioid use disorder (OUD).
A primary catalyst for the Opioid Withdrawal Treatment market is the escalating global opioid crisis. The rising incidence of opioid misuse and addiction necessitates immediate and effective treatment solutions, with withdrawal management being a critical first step. Patients experiencing withdrawal often face severe physical and psychological symptoms, making comprehensive and supportive care essential for successful long-term recovery. This pervasive public health challenge inherently drives demand for a wide array of treatment modalities, from established pharmacotherapies to emerging non-pharmacological interventions.
Technological advancements in treatment modalities are continuously shaping the Opioid Withdrawal Treatment landscape. The shift towards medication-assisted treatments (MAT), primarily involving buprenorphine (often combined with naloxone) and methadone, continues to be a cornerstone. These medications work by alleviating withdrawal symptoms and reducing cravings, significantly enhancing treatment efficacy and patient compliance. New formulations, such as long-acting injectables and implants, are being developed to improve adherence and reduce the frequency of dosing, offering greater convenience for patients and providers. Furthermore, non-pharmacological approaches, including medical devices, are gaining traction. For instance, the NET Device, which received FDA market clearance in May 2024, has shown promise in reducing opioid withdrawal symptoms through neurostimulation, offering an alternative for patients hesitant about medication-based treatments.
Increased funding and proactive government initiatives are playing a pivotal role in expanding the Opioid Withdrawal Treatment market. Governments worldwide are implementing policies aimed at broadening access to OUD treatment, often including expanded insurance coverage for medications like buprenorphine and methadone. Programs like the Mainstreaming Addiction Treatment (MAT) Act in the U.S., which eliminated the DATA-Waiver (X-Waiver) requirement for buprenorphine prescribing, have significantly increased the number of healthcare providers who can offer this vital treatment. Additionally, investments in telehealth services are enhancing accessibility, particularly for underserved populations and in rural areas, making treatment more readily available.
The complexity of opioid withdrawal, especially with the emergence of new synthetic opioids and adulterants like medetomidine in the illicit drug supply, presents both challenges and opportunities within the Opioid Withdrawal Treatment market. These novel substances can induce severe and atypical withdrawal syndromes, necessitating vigilant monitoring and potentially adjusted treatment protocols. This complexity underscores the need for continued research and development into new therapies and diagnostic tools that can rapidly identify and effectively manage such challenging withdrawal presentations. Companies and research institutions are actively exploring new therapeutic targets, with VCU researchers, for example, developing a long-acting formulation of nor-LAAM to extend the therapeutic effect of medication for opioid addiction.
North America continues to dominate the Opioid Withdrawal Treatment market, driven by the high prevalence of opioid addiction and a well-established, albeit evolving, healthcare infrastructure. However, East Asia is emerging as a rapidly growing region, indicating increasing awareness and treatment efforts in those geographies. The market segmentation covers various aspects, including treatment modalities (medications, devices, behavioral therapies), settings (inpatient, outpatient), and patient demographics, reflecting a diverse and evolving set of needs.
Despite the progress, challenges remain, including the stigma associated with OUD, barriers to treatment access in some regions, and the often chronic and relapsing nature of opioid dependence. However, the overarching opportunities, such as the growing demand for personalized treatment plans, the integration of digital health solutions, and the continuous innovation in therapeutic options, suggest a promising future for the Opioid Withdrawal Treatment market. The collaborative efforts of pharmaceutical companies, medical device manufacturers, healthcare providers, and policymakers will be crucial in expanding access to care and improving outcomes for individuals grappling with opioid addiction.